1) Francisco D, Wang Y, Conway M, Hurbon AN, Dy ABC, Addison KJ, Chu HW, Voelker DR, Ledford JG, Kraft M. Surfactant Protein-A Protects against IL-13-Induced Inflammation in Asthma. J Immunol. 2020 May 15;204(10):2829-2839. doi: 10.4049/jimmunol.1901227. Epub 2020 Apr 3. PMID: 32245819; PMCID: PMC7304346
2) Younis US, Chu HW, Kraft M, Ledford JG. A 20-Mer Peptide Derived from the Lectin Domain of SP-A2 Decreases Tumor Necrosis Factor Alpha Production during Mycoplasma pneumoniae Infection. Infect Immun. 2020 Aug 19;88(9):e00099-20. doi: 10.1128/IAI.00099-20. Print 2020 Aug 19.PMID: 32513852, PMCID: PMC7440763
3) Dy, A.B.C, Langlais, P.R., Barker, N.K., Addison, K.J., Tanyaratsrisakul, S., Boitano, S., Christenson, S.A., Kraft, M., Meyers, D., Bleecker, E.R, Li, X., Ledford, J.G. “Myeloidassociated differentiation marker is a novel SP-A-associated transmembrane protein whose expression on airway epithelial cells correlates with asthma severity.” Scientific Reports. 2021 Dec 3:11(1):23392. PMC2188087
4) Pederson WP, Cyphert-Daly JM, Tighe RM, Que LG, Ledford JG. Genetic variation in surfactant protein-A2 alters responses to ozone. PLoS One. 2021 Feb 22;16(2):e0247504. doi: 10.1371/journal.pone.0247504. eCollection, 2021. PMID: 33617569, PMCID: PMC7899376
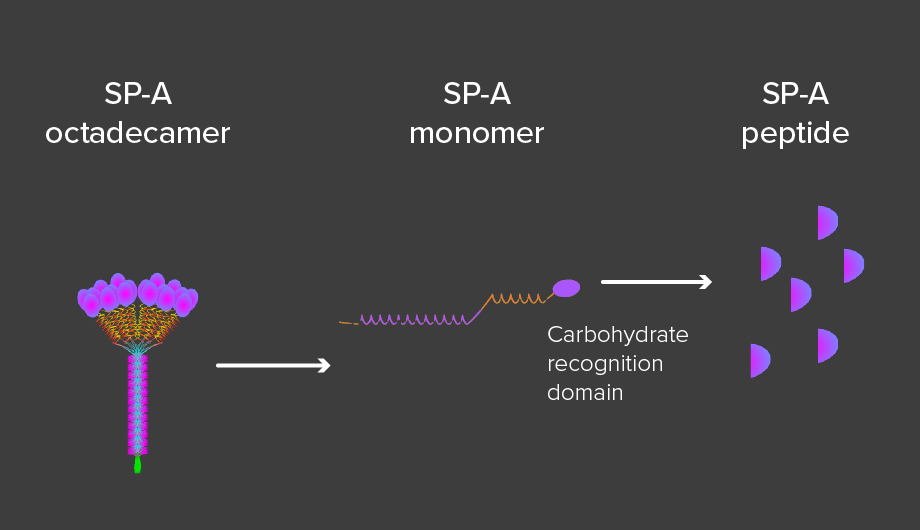
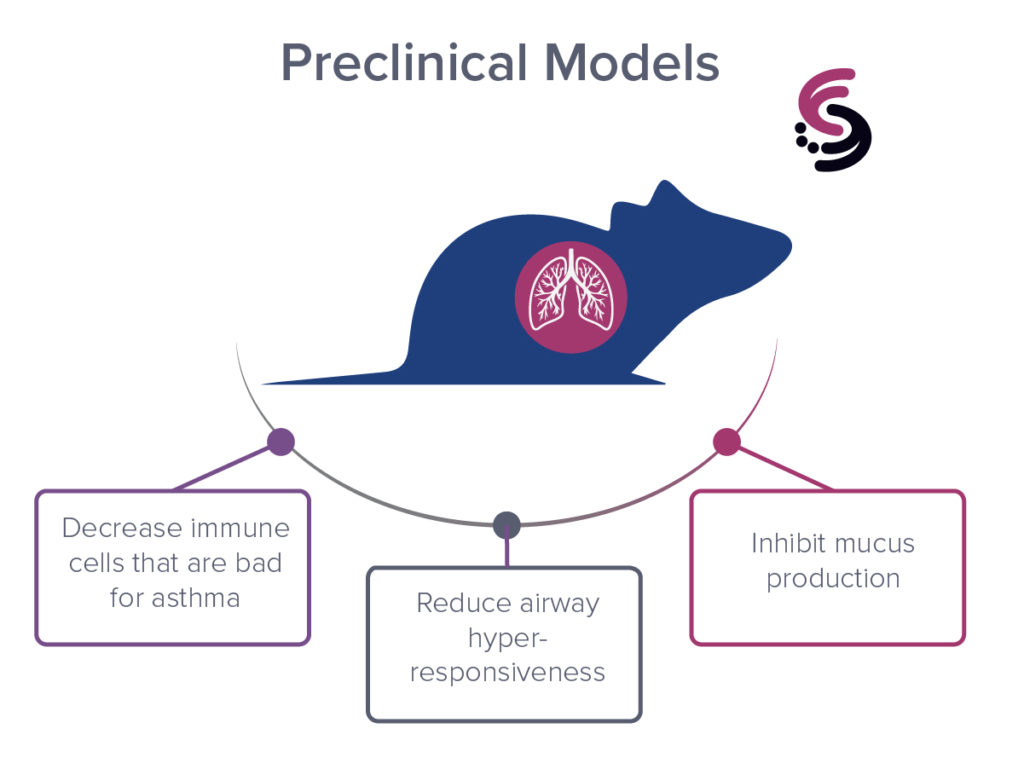
5) Francisco, D., Wang, Y., Marshall, C., Conway, M., Addison, K.J., Billheimer, D., Kumura, H., Numata, M., Chu, H.W., Voelker, D.R., 1Kraft, M, 1Ledford, J.G. Small peptide derivatives within the carbohydrate recognition domain of SP-A2 modulate asthma outcomes in mouse models and human cells. 1Co-senior authors. Frontiers Immunology, epub ahead of print, July 8, 2022.